Introduction
Reactive oxygen species (ROS) are chemically reactive molecules containing oxygen that play a dual role in cellular biology. At controlled levels, ROS participate in signaling pathways crucial for normal physiological functions such as cell growth and defense mechanisms. However, excessive ROS production leads to oxidative stress, which has been linked to cellular damage, inflammation, aging, and diseases like cancer. Understanding the dynamics of ROS within cells, particularly in fibroblasts, is essential for developing novel therapeutic strategies.
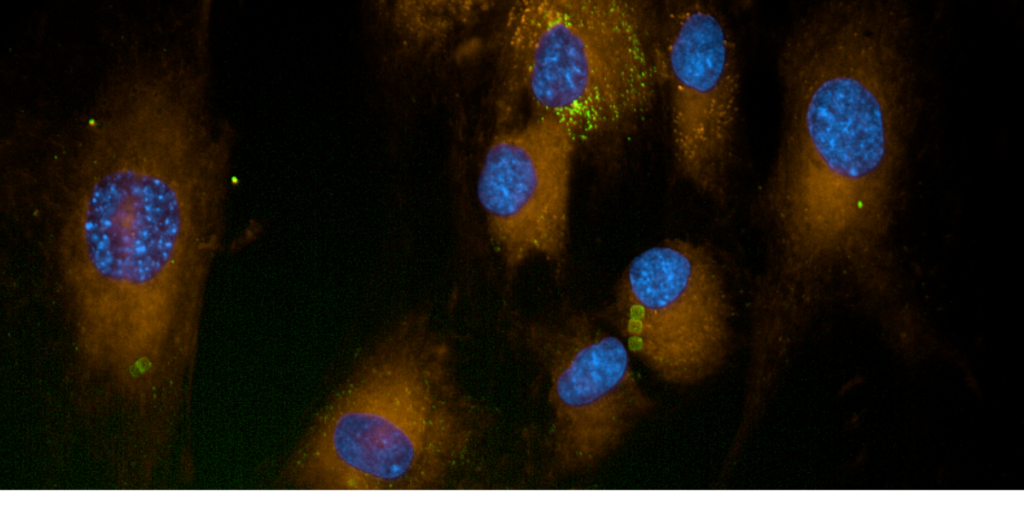
To this end, SPAchip® (Suspended Planar-Array Chips) technology has emerged as a cutting-edge tool for investigating the effects of ROS at the cellular level. The image above illustrates fibroblast cells subjected to ROS-inducing agents, with a particular focus on the visualization and analysis of ROS accumulation and distribution.
SPAchip® technology and its relevance
SPAchip is a nanotechnology-driven platform designed to monitor and manipulate the intracellular environment with extreme precision. The system integrates nanoarrays specifically tailored for biological samples, allowing real-time detection of biochemical changes such as ROS generation. The core components of SPAchip include nanoelectrodes and biosensors embedded within the chip array, providing spatial and temporal data on cellular events.
Fibroblasts, the most common type of cells found in connective tissue, are particularly susceptible to oxidative stress due to their role in producing extracellular matrix and collagen. Monitoring ROS activity in fibroblasts helps in understanding how these cells respond to oxidative insults, which has implications for tissue regeneration, wound healing, and fibrotic diseases.
ROS-Induced cellular responses in fibroblasts
1095sk fibroblast cells exposed to ROS typically exhibit a range of responses depending on the intensity and duration of oxidative stress. Acute exposure may activate repair mechanisms, such as upregulation of antioxidant enzymes (e.g., superoxide dismutase, catalase), which attempt to neutralize ROS. However, prolonged exposure can lead to:
- DNA Damage: Although not yet visible in this early-stage image, continued ROS exposure can result in nuclear DNA mutations, initiating cell death pathways or contributing to oncogenic transformation.
- Protein Oxidation: Oxidative stress can modify proteins, impairing their function. In fibroblasts, this often affects collagen production and matrix remodeling, which has downstream effects on tissue integrity and repair processes.
- Mitochondrial Dysfunction: The mitochondria are a significant source and target of ROS. Disruption in mitochondrial function due to ROS leads to energy deficits and promotes apoptosis.
SPAchip®’s role in ROS quantification
CytoCHECK SPAchip OHrad ROS allow for precise quantification of ROS at intracellular levels. This capability is essential for understanding how fibroblasts react to oxidative stress. By integrating biosensors for ROS detection directly into the chip, researchers can monitor oxidative fluctuations and correlate them with cellular damage markers.
Applications of ROS detection in fibroblasts
The ability to monitor ROS in fibroblasts using CytoCHECK SPAchip® OHrad ROS Single-Detection Kit has wide-reaching applications, particularly in the fields of regenerative medicine, drug screening, and disease modeling. Specific applications include:
- Wound healing research: understanding how fibroblasts manage oxidative stress is key to improving treatments for chronic wounds, which are often characterized by excessive ROS production and impaired fibroblast function.
- Cancer Research: since ROS levels can influence cell proliferation and mutation rates, SPAchip can be used to explore how fibroblasts contribute to the tumor microenvironment, especially in fibrotic tissues.
- Pharmacological testing: SPAchip® arrays provide an ideal platform for screening antioxidant compounds, allowing for rapid assessment of drug efficacy in reducing ROS levels and protecting fibroblasts from oxidative damage.
Conclusion
The combination of CytoCHECK SPAchip® OHrad ROS Single-Detection Kit technology with cell assays in fibroblasts provides a powerful approach to studying oxidative stress at the cellular level. The image above serves as a snapshot of the early stages of ROS-induced changes in 1095sk fibroblast morphology and biochemistry. As this technology continues to evolve, it holds the potential to revolutionize our understanding of cell-environment interactions and offers novel pathways for therapeutic development in oxidative stress-related diseases.
By leveraging advanced nanotechnology like SPAchip®, researchers are poised to make significant strides in cell biology, ultimately improving clinical outcomes in areas such as tissue repair, cancer therapy, and chronic disease management.






